how to draw amino acid sequence
Peptides & Proteins
1. The Peptide Bail
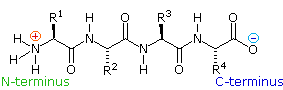
If the amine and carboxylic acid functional groups in amino acids join together to form amide bonds, a chain of amino acid units, chosen a peptide, is formed. A simple tetrapeptide construction is shown in the following diagram. By convention, the amino acid component retaining a free amine group is drawn at the left stop (the N-terminus) of the peptide chain, and the amino acid retaining a free carboxylic acid is fatigued on the right (the C-terminus). As expected, the free amine and carboxylic acrid functions on a peptide chain form a zwitterionic structure at their isoelectric pH.
By clicking the " Grow Peptide " button, an animation showing the assembly of this peptide will exist displayed. The " Prove Structure " button displays some bond angles and lengths that are characteristic of these compounds.
 |
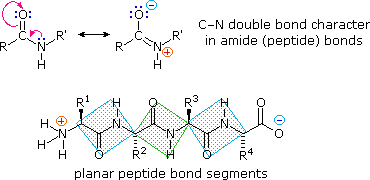
The conformational flexibility of peptide chains is limited chiefly to rotations about the bonds leading to the alpha-carbon atoms. This brake is due to the rigid nature of the amide (peptide) bond. As shown in the following diagram, nitrogen electron pair delocalization into the carbonyl group results in significant double bond character between the carbonyl carbon and the nitrogen. This keeps the peptide links relatively planar and resistant to conformational change. The color shaded rectangles in the lower construction define these regions, and place the relatively facile rotations that may accept identify where the corners meet (i.eastward. at the alpha-carbon). This aspect of peptide construction is an important factor influencing the conformations adopted by proteins and large peptides.
2. The Primary Structure of Peptides
Because the N-terminus of a peptide chain is singled-out from the C-terminus, a pocket-sized peptide composed of different aminoacids may have a several constitutional isomers. 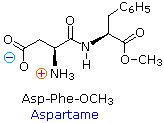 For instance, a dipeptide made from two different amino acids may have two different structures. Thus, aspartic acrid (Asp) and phenylalanine (Phe) may exist combined to brand Asp-Phe or Phe-Asp, recollect that the amino acrid on the left is the Due north-terminus. The methyl ester of the first dipeptide (structure on the correct) is the artificial sweetener aspartame, which is nearly 200 times sweeter than sucrose. Neither of the component amino acids is sweet (Phe is actually bitter), and derivatives of the other dipeptide (Phe-Asp) are not sugariness.
For instance, a dipeptide made from two different amino acids may have two different structures. Thus, aspartic acrid (Asp) and phenylalanine (Phe) may exist combined to brand Asp-Phe or Phe-Asp, recollect that the amino acrid on the left is the Due north-terminus. The methyl ester of the first dipeptide (structure on the correct) is the artificial sweetener aspartame, which is nearly 200 times sweeter than sucrose. Neither of the component amino acids is sweet (Phe is actually bitter), and derivatives of the other dipeptide (Phe-Asp) are not sugariness.
A tripeptide composed of iii different amino acids can exist made in half dozen different constitutions, and the tetrapeptide shown above (composed of four different amino acids) would accept 24 ramble isomers. When all twenty of the natural amino acids are possible components of a peptide, the possible combinations are enormous. Simple statistical probability indicates that the decapeptides made up from all possible combinations of these amino acids would total xx10!
Natural peptides of varying complexity are arable. The simple and widely distributed tripeptide glutathione (showtime entry in the post-obit table), is interesting because the side-chain carboxyl part of the N-terminal glutamic acid is used for the peptide bail. An Due north-terminal glutamic acid may also close to a lactam ring, equally in the instance of TRH (2d entry). The abbreviation for this transformed unit is pGlu (or pE), where p stands for "pyro" (such band closures often occur on heating). The larger peptides in the table also demonstrate the importance of amino acid abbreviations, since a full structural formula for a nonapeptide (or larger) would evidence to be complex and unwieldy. The formulas using single letter abbreviations are colored red.
The 10 peptides listed in this table make use of all twenty common amino acids. Note that the C-final unit of measurement has the form of an amide in some cases (eastward.yard. TRH, angiotensin & oxytocin). When two or more cysteines are present in a peptide concatenation, they are oftentimes joined by disulfide bonds (eastward.g. oxytocin & endothelin); and in the case of insulin, two separate peptide chains (A & B) are held together past such links.
| Name (residues) | Source or Office | Amino Acrid Sequence |
|---|---|---|
| Glutathione (iii) | Most Living Cells (stimulates tissue growth) | (+)H3NCH(CO2 (–))CH2CHiiCONHCH(CH2SH)CONHCHiiCO2H γ-Glu-Cys-Gly (or γECG) |
| TRH (three) | Hypothalmic Neurohormone (governs release of thyrotropin) | 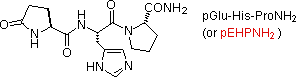 |
| Angiotensin Ii (8) | Pressor Amanuensis (acts on the adrenal gland) | Asp-Arg-Val-Tyr-Ile-His-Pro-PheNH2 (or DRVYIHPFNHtwo ) |
| Bradykinen (9) | Hypotensive Vasodilator (acts on smoothen muscle) | Arg-Pro-Pro-Gly-Phe-Ser-Pro-Phe-Arg (or RPPGFSPFR) |
| Oxytocin (9) | Uterus-Contracting Hormone (also stimulates lactation) |  |
| Somatostatin (14) | Inhibits Growth Hormone Release (used to treat ulcers) |  |
| Endothelin (21) | Potent Vasoconstrictor (structurally similar to some snake venoms) |  |
| Melittin (26) | Honey Bee Venom (used to treat rheumatism) | Gly-Ile-Gly-Ala-Val-Leu-Lys-Val-Leu-Thr-Thr-Gly-Leu-Pro~ ~Ala-Leu-Ile-Ser-Trp-Ile-Lys-Arg-Lys-Arg-Gln-GlnNHtwo (or GIGAVLKVLTTGLPALISWIKRKRQQNHtwo ) |
| Glucagon (29) | Hyperglycemic Factor (used every bit an anti-diabetic) | His-Ser-Gln-Gly-Thr-Phe-Thr-Ser-Asp-Tyr-Ser-Lys-Tyr-Leu-Asp~ ~Ser-Arg-Arg-Ala-Gln-Asp-Phe-Val-Gln-Trp-Leu-Met-Asn-Thr (or HSQGTFTSDYSKYLDSRRAQDFVQWLMNT) |
| Insulin (51) | Pancreatic Hormone (used in treatment of diabetes) |  |
The different amino acids that brand up a peptide or protein, and the order in which they are joined together by peptide bonds is referred to equally the chief construction. From the examples shown above, it should exist evident that it is not a trivial chore to determine the primary structure of such compounds, even modestly sized ones.
Complete hydrolysis of a protein or peptide, followed by amino acid analysis establishes its gross composition, but does non provide any bonding sequence information.
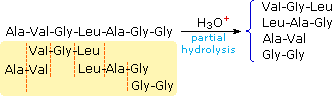
Fractional hydrolysis volition produce a mixture of shorter peptides and some amino acids. If the main structures of these fragments are known, it is sometimes possible to deduce part or all of the original structure by taking advantage of overlapping pieces. For case, if a heptapeptide was equanimous of three glycines, two alanines, a leucine and a valine, many possible principal structures could be written. On the other hand, if partial hydrolysis gave 2 known tripeptide and two known dipeptide fragments, as shown on the right, uncomplicated assay of the overlapping units identifies the original primary structure. Of course, this kind of structure determination is very inefficient and unreliable. First, we demand to know the structures of all the overlapping fragments. Second, larger peptides would give circuitous mixtures which would have to be separated and painstakingly examined to notice suitable pieces for overlapping. It should exist noted, however, that modernistic mass spectrometry uses this overlap technique effectively. The difference is that bond cleavage is non accomplished by hydrolysis, and computers assume the fourth dimension consuming chore of comparing a multitude of fragments.
3. North-Terminal Group Analysis
Over the years that chemists have been studying these important natural products, many techniques have been used to investigate their primary structure or amino acid sequence. Indeed, commercial instruments that automatically sequence peptides and proteins are at present available. A few of the most of import and normally used techniques will exist described here.
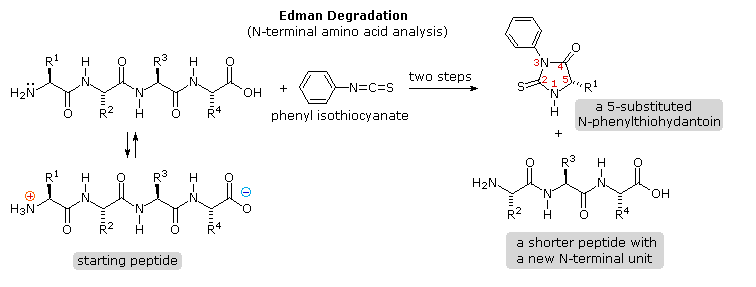
Identification of the Northward-terminal and C-terminal aminoacid units of a peptide chain provides helpful information. N-final analysis is accomplished by the Edman Degradation, which is outlined in the following diagram. A free amine office, unremarkably in equilibrium with zwitterion species, is necessary for the initial bonding to the phenyl isothiocyanate reagent. The products of the Edman deposition are a thiohydantoin heterocycle incorporating the Northward-last amino acid together with a shortened peptide chain. Amine functions on a side-chain, equally in lysine, may react with the isothiocyanate reagent, but do non give thiohydantoin products.
 |
Repeated clicking of the " Next Diagram " button displays the machinery of this important analytical method.
A major advantage of the Edman procedure is that the remaining peptide chain is non further degraded past the reaction. This means that the N-final analysis may be repeated several times, thus providing the sequence of the first three to five amino acids in the chain. A disadvantage of the procedure is that is peptides larger than 30 to 40 units practise not give reliable results.
4. C-Final Group Assay
Chemical Analysis
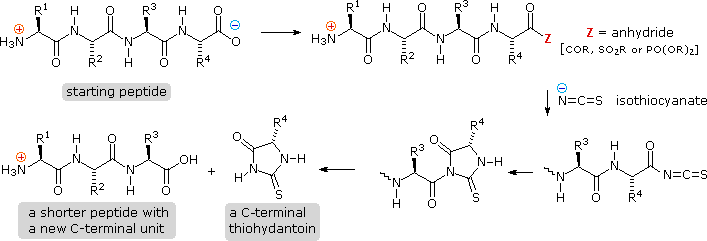
Complementary C-terminal analysis of peptide chains may be achieved chemically or enzymatically. The chemical analysis is slightly more circuitous than the Edman procedure. Get-go, side-chain carboxyl groups and hydroxyl groups must be protected as amides or esters. Side by side, the C-terminal carboxyl group is activated as an anhydride and reacted with thiocyanate. The resulting acyl thiocyanate immediately cyclizes to a hydantoin ring, and this can be cleaved from the peptide chain in several ways, not described here. Depending on the nature of this final cleavage, the procedure can exist modified to give a C-terminal acyl thiocyanate peptide product which automatically rearranges to a thiohydantoin incorporating the penultimate C-terminal unit of measurement. Thus, repetitive analyses may be conducted in much the same way they are with the Edman procedure.
Enzymatic Analysis
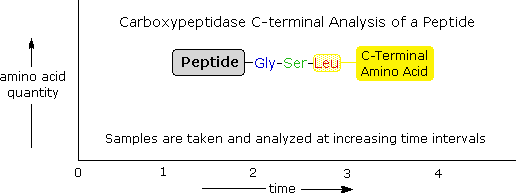
Enzymatic C-terminal amino acrid cleavage by one of several carboxypeptidase enzymes is a fast and convenient method of assay. Because the shortened peptide product is too subject to enzymatic cleavage, care must exist taken to control the conditions of reaction and so that the products of successive cleavages are properly monitored. The post-obit example illustrates this feature. A peptide having a C-terminal sequence: ~Gly-Ser-Leu is subjected to carboxypeptidase cleavage, and the free aminoacids broken in this reaction are analyzed at increasing time intervals. By clicking on the diagram, the results of this experiment will be displayed. The leucine is broken outset, the serine second, and the glycine third, as demonstrated by the sequential analysis. Of course, fourth and 5th units will likewise be released as time passes, merely these products are not shown.
5. Selective Peptide Cleavage
| Proper noun | Type | Specificity |
|---|---|---|
| Cyanogen Bromide | Chemical | Carboxyl Side of Methionine |
| Trypsin | Enzymatic | Carboxyl Side of Basic Amino Acids e.grand. Lys & Arg |
| Chymotrypsin | Enzymatic | Carboxyl Side of Aryl Amino Acids e.g. Phe, Tyr & Trp |
Mechanisms for the enzymatic reactions are not every bit easily formulated. Other enzymatic cleavages accept been adult, but the two listed here will serve to illustrate their application.
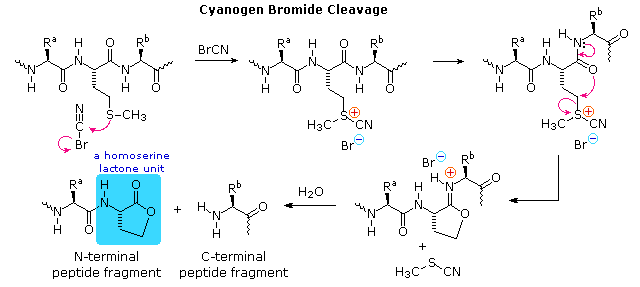
An Example of Master Structure Analysis
To see how these procedures can be combined to elucidate the primary structure of a peptide, consider the melanocyte stimulating hormone isolated from pigs. This octadecapeptide (18 amino acrid units) has the composition: Arg,Asp2,Glu2,Glyii,His,Lys2,Met,Phe,Pro3,Ser,Tyrtwo, and is abbreviated P18 . The post-obit diagram, which begins with the results of terminal unit of measurement assay, illustrates the logical steps that could be used to solve the structural problem. By clicking the "Next Phase" push button the results and conclusions from each step will exist displayed. Comments about each stage are presented nether the diagram.
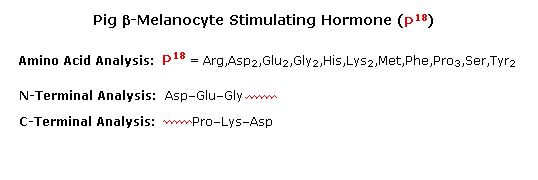 |
a) Cyanogen bromide cleavage gives two peptide fragments, the longer of which has all the units on the C-final side of methionine.
b) N-terminal analysis of the undecapeptide fragment, P11 , locates the 3 amino acids to the right of methionine.
c) Trypsin cleavage of P11 shows the location of the single arginine, which is found as the C-terminal unit of the tetrapeptide fragment. I of the two lysines was known to be next to the C-terminus. The other must be part of the smaller peptide from the cyanogen bromide reaction.
d) With only four amino acids remaining to exist located, the position of the second tyrosine may be pursued by chymotrypsin cleavage of P18 itself. Four fragments are obtained, and the final structure might have been solved by these alone. Notwithstanding, selective last grouping analysis of the two pentapeptides serves to locate the tyrosine and a second proline adjacent to the left most glycine, as well every bit identifying the units on each side of the methionine. The one remaining amino acid, a proline, is then placed at the last vacant site (yellow box).
vi. Cyclic Peptides
If the carboxyl part at the C-terminus of a peptide forms a peptide bond with the Due north-terminal amine group a cyclic peptide is formed. Carboxyate and amine functions on side chains may as well combine to form rings. Circadian peptides are most commonly plant in microorganisms, and often incorporate some D-amino acids as well every bit unusual amino acids such as ornithine (Orn). The decapeptide antibiotic gramacidin S, produced by a strain of Bacillus brevis, is one example of this interesting grade of natural products. The structure of gramicidin S is shown in the following diagram. The atypical amino acids are colored. When using a shorthand annotation for cyclic structures, the summit line is written by the usual convention (N-group on the left), but vertical and lower lines must be adjusted to fit the bonding. Arrows on these bonds point in the CO-Due north direction of each peptide bond.
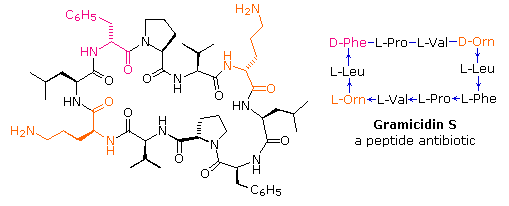
To see a model of another cyclic peptide, having potentially useful medicinal properties Click Here.
Structure-Property Relationships
The compounds we call proteins exhibit a wide range of concrete and biological properties. 2 general categories of elementary proteins are ordinarily recognized.
| Fibrous Proteins | As the name implies, these substances accept cobweb-like structures, and serve equally the chief structural material in various tissues. Corresponding to this structural function, they are relatively insoluble in water and unaffected by moderate changes in temperature and pH. Subgroups within this category include: Collagens & Elastins, the proteins of connective tissues. tendons and ligaments. Keratins, proteins that are major components of skin, hair, feathers and horn. Fibrin, a protein formed when claret clots. | |
|---|---|---|
| Globular Proteins | Members of this class serve regulatory, maintenance and catalytic roles in living organisms. They include hormones, antibodies and enzymes. and either deliquesce or form colloidal suspensions in water. Such proteins are by and large more sensitive to temperature and pH change than their fibrous counterparts. More than Information |
1. The Secondary and Third Construction of Large Peptides and Proteins
The various backdrop of peptides and proteins depend not only on their component amino acids and their bonding sequence in peptide bondage, but also on the fashion in which the peptide chains are stretched, coiled and folded in space. Considering of their size, the orientational options open to these macromolecules might seem well-nigh infinite. Fortunately, several factors act to narrow the structural options, and it is possible to identify some mutual structural themes or secondary structures that appear repeatedly in unlike molecules. These conformational segments are sometimes described by the dihedral angles Φ & Ψ, defined in the diagram on the right below. Most proteins and large peptides practise not adopt completely uniform conformations, and full descriptions of their preferred three dimensional arrangements are defined equally third structures.
| 5 factors that influence the conformational equilibria of peptide chains are: | 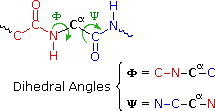 |
A. Helical Coiling
The relatively simple undecapeptide shown in the following diagram can prefer a zig-zag linear conformation, every bit drawn. A ball & stick model of this peptide volition exist displayed by clicking the appropriate button. However, this molecule prefers to assume a coiled helical conformation, displayed by clicking any of the three buttons on the right. The middle button shows a stick model of this helix, with the backbone chain drawn as a heavy black line and the hydrogen bonds as dashed maroon lines. The other buttons display a ball & stick model and a ribbon that defines this α-helix. 7 hydrogen bonds, that together provide roughly xxx kcal/mol stability, assist to maintain this conformation.
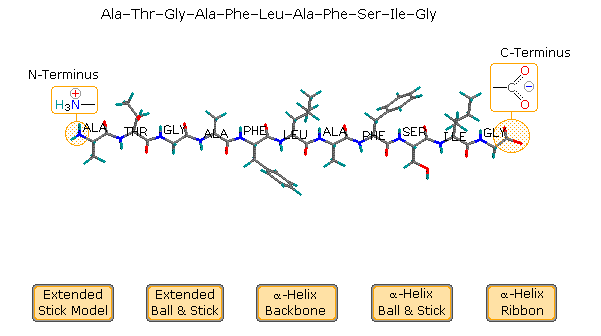
Examine the drawing activated past the centre push button. The N-terminal residue (Ala) is on the left, and the C-terminal Gly on the right. The blastoff-helix is correct-handed, which means that it rotates clockwise equally it spirals away from a viewer at either stop. Other structural features that define an alpha-helix are: the relative locations of the donor and acceptor atoms of the hydrogen bond, the number of amino acid units per helical plough and the distance the turn occupies forth the helical axis. The offset hydrogen bond (from the N-terminal cease) is from the carbonyl group of the alanine to the Due north-H group of the phenylalanine. Three amino acids, Thr, Gly & Ala, fall entirely inside this plow. Parts of the N-terminal alanine acceptor and the phenylalanine donor also fall within this helical turn, and careful analysis of the structure indicates there are three.half dozen amino acid units per turn. The altitude covered by the turn is v.4 Å. Using the dihedral angle terminology noted above, a perfect α-helix has Φ = -58º and Ψ = -47º. In natural proteins the values associated with α-helical conformations range from -57 to -70º for Φ, and from -35 to -48º for Ψ. To examine a model of this alpha-helix, click on the green circle. Once this display is activated, the of import hormone insulin may exist shown by clicking the appropriate push button in the blueish-shaded rows.
Helical conformations of peptide chains may as well be described past a 2 number term, nm , where northward is the number of amino acid units per turn and 1000 is the number of atoms in the smallest ring divers by the hydrogen bail. Using this terminology, the alpha-helix is a 3.613 helix. Other common helical conformations are 310 and 4.416. The alpha helix is the about stable of these, accounting for a tertiary of the secondary structure found in most globular (non-fibrous) proteins.
B. β-Pleated Sheets
The linear zig-zag conformation of a peptide chain may be stabilized by hydrogen bonding to adjacent parallel chains of the same kind. Beefy side-chain substituents destabilize this organization due to steric crowding, so this beta-canvass conformation is usually express to peptides having a large amount of glycine and alanine. Steric interactions also cause a slight bending or contraction of the peptide chains, and this results in a puckered distortion (the pleated canvas). As shown in the post-obit diagram, the adjacent chains may be oriented in contrary North to C directions, termed antiparallel. Using the dihedral bending terminology, an antiparallel β-sail has Φ = -139º and a Ψ = 135º. Alternatively, the adjacent peptide chains may exist oriented in the same direction, termed parallel. By convention, beta-sheets are designated by broad arrows or cartoons, pointing in the direction of the C-terminus. In this diagram, these cartoons (colored violet) are displayed by clicking on the advisable button. A model of a ii-antiparallel-chain construction may be examined by clicking on the green circle.
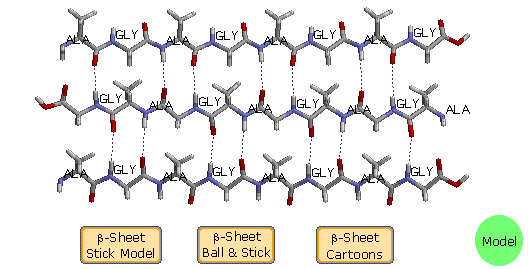
Some proteins accept layered stacks of β-sheets, which impart structural integrity and may open to form a cavity (a beta butt). An instance is human retinol bounden protein, which has a crenel formed by eight β-sheet strands. A model of this interesting protein may exist displayed by clicking the upper button in the blue-shaded rows.
When beta-sheets are observed equally secondary structural components of globular proteins, they are twisted past about 5 to 25º per residue; consequently, the planes of the sheets are non parallel. The twist is always of the same handedness, and is usually greater for antiparallel sheets. Examples will be plant in the following structures.
C. Other Structures
Although nearly proteins and large peptides may have alpha-helix and beta-sail segments, their tertiary structures may consist of less highly organized turns, strands and coils. Turns opposite the direction of the peptide chain, and are considered to be a third mutual secondary construction motif. Approximately a third of all the residues in globular proteins are plant in turns. Turns occur chiefly on the protein surface, often contain polar and charged residues, and accept been classified in iii sub-groups.
As noted earlier, several factors perturb the organization of peptide bondage. One that has not yet been cited is the structural influence of proline. Different the other common amino acids, rotation about the α C-Northward bond in proline is non possible due to the structural constraint of the five-membered ring. Consequently, the presence of a proline in a peptide chain introduces a bend or kink that disrupts helices or sheets. Also, prolines that are function of a peptide chain have no N-H hydrogen bonding donors to contribute to conformer stabilization.
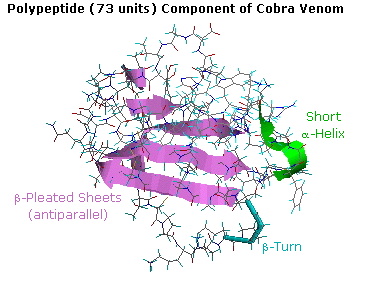
With the exception of silk fibroin and certain synthetically engineered peptides, significant portions of most proteins prefer conformations that resist simple description or categorization. For example, the post-obit diagram shows the tertiary structure of a polypeptide neurotoxin found in cobra venom. A large section of antiparallel beta-sheets is colored violet, and a brusque alpha-helix is greenish. The remaining peptide chain seems disorganized, but certain features such as a 180º turn (called a beta-turn) and five disulfide bonds can exist identified. A Chime model of this compound may be examined by clicking on the diagram.
Additional Examples
A full description and give-and-take of protein structure is beyond the telescopic of this text, only a few boosted examples will be instructive. In improver to the tertiary structures that will be displayed, attention must also be given to the way in which peptide structures may aggregate to grade dimeric, trimeric and tetrameric clusters. These assemblies, known as quaternary structures, have characteristic properties different from their monomeric components. The examples of mellitin, collagen and hemoglobin, shown below demonstrate this feature.
Some proteins incorporate nonpeptide molecules in their overall structure, either bonded covalently or positioned by other forces. These are called conjugated proteins, and the not-peptide components are referred to as prosthetic groups. Examples of conjugated proteins include:
Glycoproteins, incorporating polysaccharide prosthetic groups (e.g. collagen and fungus).
Lipoproteins, incorporating lipid prosthetic groups (eastward.g. HDL and LDL).
Chromoproteins, incorporating colored prosthetic groups (east.g. hemoglobin).
The seven illustrations shown beneath identify a ready of peptides and proteins that may be examined as Jmol models by clicking on a selected flick.
Endothelin & Angiogenin are minor peptides that have important and selective physiological properties.
Lysozyme a typical globular protein, incorporating many identifiable secondary structures.
Mellitin, from honey bee venom, has a well-defined fourth structure, half of which is shown here.
Collagen is a widely distributed fibrous protein with a large and complex quaternary structure. Only a small model segment is shown here.
Thioredoxin is a relatively small regulatory protein serving an important redox office.
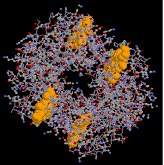
Hemoglobin, the almost complex of these examples, is a big conjugated poly peptide that transports oxygen. A 170 pound man has about a kilogram of hemoglobin distributed amidst some five billion red blood cells. A liter of arterial claret at trunk temperature can send over 200 mL of oxygen, whereas the aforementioned fluid stripped of its hemoglobin volition carry simply two to 3 mL. The supramolecular assembly of iv subunits exemplifies a quaternary structure.
 | 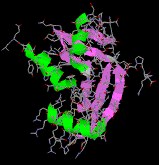 | 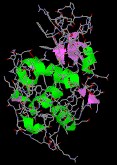 |  |
| endothelin | angiogenin | lysozyme | mellitin |
|---|
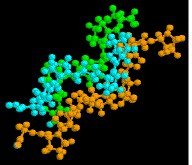 | 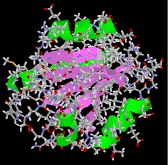 |  |
| collagen | thioredoxin | hemoglobin |
|---|
two. Fourth Structures of Proteins
Many proteins are actually assemblies of several polypeptides, which in the context of the larger aggregate are known as protein subunits. Such multiple-subunit proteins possess a 4th structure, in addition to the tertiary construction of the subunits. The subunits of a quaternary structure are held together by the aforementioned forces that are responsible for tertiary structure stabilization. These include hydrophobic attraction of nonpolar side chains in contact regions of the subunits, electrostatic interactions betwixt ionic groups of opposite charge: hydrogen bonds between polar groups; and disulfide bonds. Examples of proteins having a quaternary (or quartary) structure include hemoglobin, HIV-i protease and the insulin hexamer.
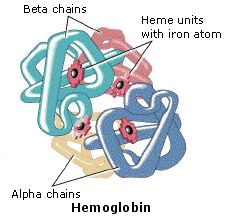 The hemoglobin molecule is an associates of four poly peptide subunits, 2 alpha units and two beta units. Each protein chain folds into a set of alpha-helix structural segments continued together in a globin arrangement, so called because this arrangement is the same folding motif used in other heme/globin proteins such as myoglobin. This folding blueprint contains a pocket which strongly binds the heme grouping. The 4 polypeptide chains are bound to each other by common salt bridges, forming a tetrameric quaternary construction. A model of hemoglobin was shown above, and may too be examined by clicking the image on the left.
The hemoglobin molecule is an associates of four poly peptide subunits, 2 alpha units and two beta units. Each protein chain folds into a set of alpha-helix structural segments continued together in a globin arrangement, so called because this arrangement is the same folding motif used in other heme/globin proteins such as myoglobin. This folding blueprint contains a pocket which strongly binds the heme grouping. The 4 polypeptide chains are bound to each other by common salt bridges, forming a tetrameric quaternary construction. A model of hemoglobin was shown above, and may too be examined by clicking the image on the left.
In animals, hemoglobin transports oxygen from the lungs or gills to the remainder of the body, where information technology releases the oxygen for cell utilize. Hemoglobin's oxygen-binding capacity is decreased in the presence of carbon monoxide because both gases compete for the aforementioned binding sites on hemoglobin. The binding affinity of hemoglobin for CO is 200 times greater than its affinity for oxygen. When hemoglobin combines with CO, information technology forms a very bright ruddy compound called carboxyhemoglobin, which may crusade the skin of CO poisoning victims to appear pink in death. In heavy smokers, up to twenty% of the oxygen-active sites tin can be blocked by CO. Similarly, hemoglobin has a competitive binding affinity for cyanide, sulfur monoxide, nitrogen dioxide and sulfides including hydrogen sulfide . All of these bind to the heme iron without changing its oxidation state, causing grave toxicity.
Insulin is a peptide hormone composed of 51 amino acids, with a molecular weight of 5808 Da. Insulin has a potent upshot on metabolism and other torso functions, causing cells in the liver, muscle, and fatty tissue to take up glucose from the claret, storing information technology as glycogen in the liver and muscle. Insulin is formed in the islets of Langerhans in the pancreas.
The molecular structure of insulin varies slightly between species of animals. Porcine (pig) insulin is especially close to the man version. Insulin molecules have a tendency to grade dimers in solution due to hydrogen-bonding between the C-termini of B chains. In the presence of zinc ions, insulin dimers acquaintance into hexamers. Insulin is stored in the trunk as a hexamer, whereas the active form is the monomer. These interactions have important clinical ramifications. Monomers and dimers readily diffuse into claret; hexamers diffuse poorly. Past clicking the epitome on the far left, a model of the insulin monomer will be displayed . A model of the hexamer volition be shown by clicking its paradigm.
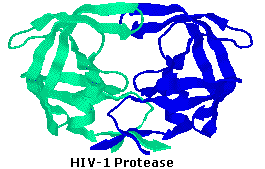 HIV-1 protease is an enzyme made by the HIV virus that is essential for it's life-wheel. The virus makes certain proteins that need to be cleaved or cutting, in club to transform into functional proteins that enable the virus to infect new cells. HIV-i protease cleaves the nascent proteins into their functional form. The enzyme is composed of 2 symmetrically related subunits, shown here in cartoon backbone representation to highlight the secondary structure. Each subunit consists of the same small chain of 99 amino acids, which come up together in such as way equally to form a tunnel where they meet. The protein to be cleaved sits in this tunnel, which houses the active site of the enzyme. Ii Asp-Thr-Gly catalytic triads, 1 on each chain, etch the active site. The two Asp's act as the primary catalytic agents, and together with a h2o molecule carve the protein chain spring in the tunnel. Without constructive HIV-ane protease, HIV virions remain uninfectious.
HIV-1 protease is an enzyme made by the HIV virus that is essential for it's life-wheel. The virus makes certain proteins that need to be cleaved or cutting, in club to transform into functional proteins that enable the virus to infect new cells. HIV-i protease cleaves the nascent proteins into their functional form. The enzyme is composed of 2 symmetrically related subunits, shown here in cartoon backbone representation to highlight the secondary structure. Each subunit consists of the same small chain of 99 amino acids, which come up together in such as way equally to form a tunnel where they meet. The protein to be cleaved sits in this tunnel, which houses the active site of the enzyme. Ii Asp-Thr-Gly catalytic triads, 1 on each chain, etch the active site. The two Asp's act as the primary catalytic agents, and together with a h2o molecule carve the protein chain spring in the tunnel. Without constructive HIV-ane protease, HIV virions remain uninfectious.
Because of its part in HIV replication, HIV-1 protease has been a target for antiviral drugs. Such drugs role as inhibitors, binding to the active site by mimicking the tetrahedral intermediate of its substrate, thus disabling the enzyme. The structure of 1 such inhibitor, BEA388, volition be displayed on the left by clicking hither.
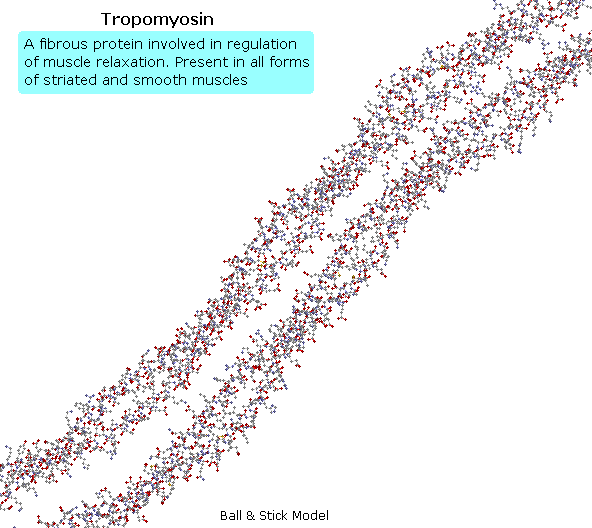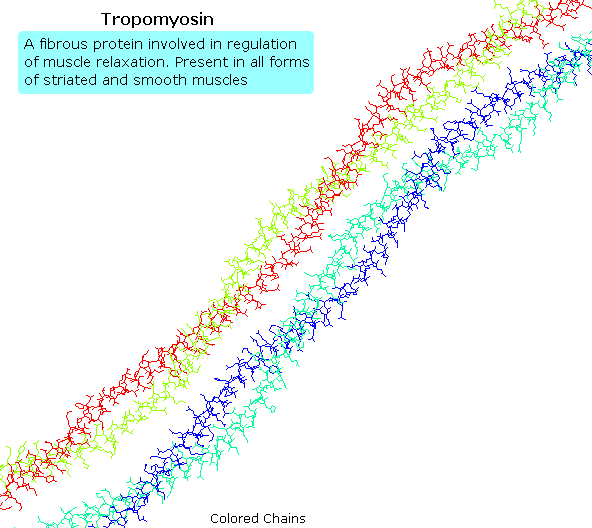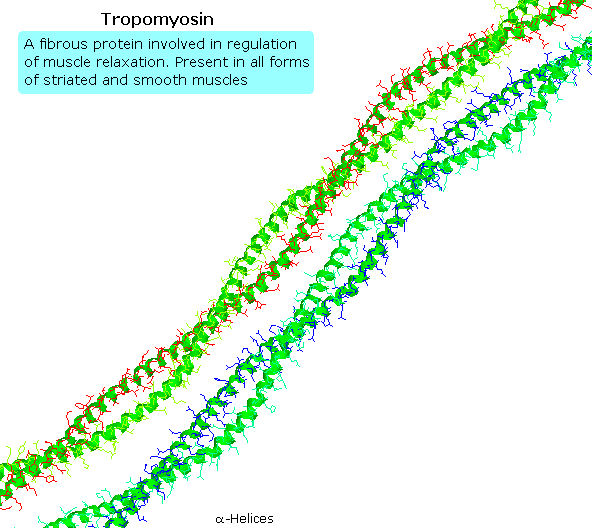
Tropomyosin
The following blitheness shows a segment of the fibrous protein tropomyosin, a common muscle regulator. The peptide chains are largely alpha-helices. These are wrapped in superhelix pairs, which are and then aligned in a parallel array.If animation is non occurring, click on the drawing or reload.
Peptide Synthesis
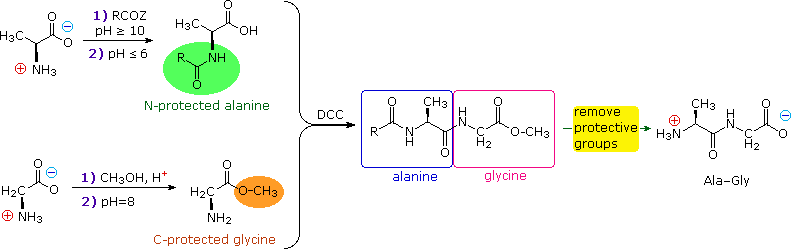
In order to synthesize a peptide from its component amino acids, two obstacles must be overcome. The first of these is statistical in nature, and is illustrated by considering the dipeptide Ala-Gly as a proposed target. If we ignore the chemical science involved, a mixture of equal molar amounts of alanine and glycine would generate four different dipeptides. These are: Ala-Ala, Gly-Gly, Ala-Gly & Gly-Ala. In the instance of tripeptides, the number of possible products from these two amino acids rises to eight. Clearly, some kind of selectivity must be exercised if complex mixtures are to exist avoided.
The second difficulty arises from the fact that carboxylic acids and 1º or 2º-amines do not class amide bonds on mixing, merely will generally react by proton transfer to give salts (the intermolecular equivalent of zwitterion formation).
From the perspective of an organic chemist, peptide synthesis requires selective acylation of a free amine. To accomplish the desired amide bond germination, we must first deactivate all extraneous amine functions so they do not compete for the acylation reagent. And then nosotros must selectively activate the designated carboxyl part so that information technology will acylate the one remaining gratuitous amine. Fortunately, chemical reactions that permit united states to accomplish these selections are well known.
First, the basicity and nucleophilicity of amines are substantially reduced by amide germination. Consequently, the acylation of amino acids by treatment with acyl chlorides or anhydrides at pH > x, as described before, serves to protect their amino groups from further reaction.
Second, acyl halide or anhydride-like activation of a specific carboxyl reactant must occur as a prelude to peptide (amide) bail formation. This is possible, provided competing reactions involving other carboxyl functions that might be present are precluded by preliminary ester formation. Remember, esters are weaker acylating reagents than either anhydrides or acyl halides, as noted before.
Finally, dicyclohexylcarbodiimide (DCC) furnishings the aridity of a carboxylic acid and amine mixture to the corresponding amide under relatively mild conditions. The structure of this reagent and the mechanism of its activeness accept been described. Its application to peptide synthesis will become apparent in the following discussion.
The strategy for peptide synthesis, as outlined here, should now be apparent. The post-obit example shows a selective synthesis of the dipeptide Ala-Gly.
An important issue remains to be addressed. Since the N-protective group is an amide, removal of this part might require conditions that would likewise cleave the simply formed peptide bail. Furthermore, the harsh conditions often required for amide hydrolysis might cause extensive racemization of the amino acids in the resulting peptide. This trouble strikes at the heart of our strategy, so it is important to requite careful thought to the design of specific N-protective groups. In detail, three qualities are desired:
1) The protective amide should be easy to attach to amino acids.
ii) The protected amino group should non react under peptide forming conditions.
three) The protective amide group should be easy to remove nether mild conditions.
A number of protective groups that satisfy these conditions accept been devised; and 2 of the nearly widely used, carbobenzoxy (Cbz) and t-butoxycarbonyl (BOC or t-BOC), are described hither.
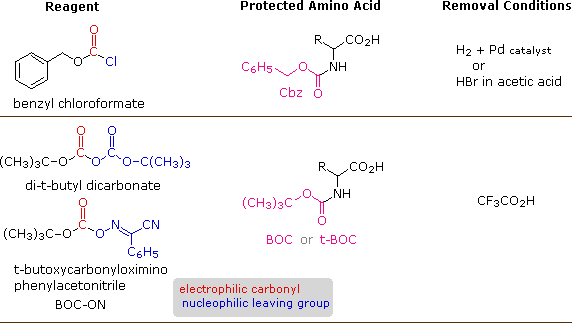
The reagents for introducing these N-protective groups are the acyl chlorides or anhydrides shown in the left portion of the higher up diagram. Reaction with a free amine function of an amino acid occurs rapidly to give the "protected" amino acid derivative shown in the eye. This can so be used to class a peptide (amide) bond to a 2nd amino acid. Once the desired peptide bond is created the protective grouping tin can exist removed under relatively mild non-hydrolytic conditions. Equations showing the protective grouping removal will be displayed to a higher place by clicking on the diagram. Cleavage of the reactive benzyl or tert-butyl groups generates a mutual carbamic acid intermediate (HOCO-NHR) which spontaneously loses carbon dioxide, giving the corresponding amine. If the methyl ester at the C-terminus is left in place, this sequence of reactions may be repeated, using a different N-protected amino acid as the acylating reagent. Removal of the protective groups would and so yield a specific tripeptide, adamant by the nature of the reactants and lodge of the reactions.
The synthesis of a peptide of meaning length (east.g. x residues) by this approach requires many steps, and the product must be advisedly purified after each step to preclude unwanted cross-reactions. To facilitate the ho-hum and fourth dimension consuming purifications, and reduce the material losses that occur in treatment, a clever modification of this strategy has been developed. This procedure, known every bit the Merrifield Synthesis after its inventor R. Bruce Merrifield, involves attaching the C-terminus of the peptide chain to a polymeric solid, ordinarily having the form of very minor chaplet. Separation and purification is only achieved by filtering and washing the beads with advisable solvents. The reagents for the next peptide bond addition are then added, and the purification steps repeated. The entire procedure can exist automated, and peptide synthesis machines based on the Merrifield approach are commercially bachelor. A series of equations illustrating the Merrifield synthesis may exist viewed by clicking on the following diagram. The terminal pace, in which the completed peptide is released from the polymer support, is a unproblematic benzyl ester cleavage. This is not shown in the display.
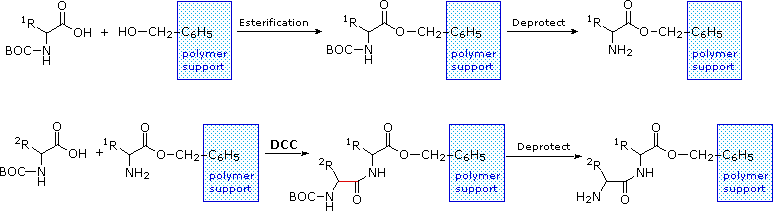 |
Two or more than moderately sized peptides can be joined together past selective peptide bond formation, provided side-concatenation functions are protected and exercise not interfere. In this fashion adept sized peptides and small-scale proteins may be synthesized in the laboratory. However, even if chemists assemble the principal structure of a natural poly peptide in this or any other fashion, it may non immediately prefer its native secondary, third and fourth structure. Many factors, such every bit pH, temperature and inorganic ion concentration influence the conformational coiling of peptide chains. Indeed, scientists are still trying to understand how and why these college structures are established in living organisms.
Denaturation
The natural or native structures of proteins may exist altered, and their biological activity changed or destroyed by treatment that does not disrupt the chief structure. This denaturation is often done deliberately in the class of separating and purifying proteins. For example, many soluble globular proteins precipitate if the pH of the solution is set at the pI of the protein. Also, addition of trichloroacetic acid or the bis-amide urea (NH2CONH2) is commonly used to effect protein precipitation. Following denaturation, some proteins will render to their native structures under proper conditions; but farthermost conditions, such equally strong heating, usually cause irreversible change.
Some treatments known to denature proteins are listed in the following table.
Denaturing Action | Machinery of Operation |
|---|---|
| Rut | hydrogen bonds are broken past increased translational and vibrational energy. (coagulation of egg white albumin on frying.) |
| Ultraviolet Radiations | Like to oestrus (sunburn) |
| Strong Acids or Bases | table salt formation; disruption of hydrogen bonds. (peel blisters and burns, protein precipitation.) |
| Urea Solution | competition for hydrogen bonds. (precipitation of soluble proteins.) |
| Some Organic Solvents | change in dielectric constant and hydration of ionic groups. (disinfectant action and precipitation of poly peptide.) |
| Agitation | shearing of hydrogen bonds. (beating egg white albumin into a meringue.) |
Not all proteins are easily denatured. As noted above, fibrous proteins such as keratins, collagens and elastins are robust, relatively insoluble, fourth structured proteins that play important roles in the physical structure of organisms. Secondary structures such equally the α-helix and β-sail take on a dominant role in the compages and aggregation of keratins. In add-on to the intra- and intermolecular hydrogen bonds of these structures, keratins have large amounts of the sulfur-containing amino acid Cys, resulting in disulfide bridges that confer additional strength and rigidity. The more flexible and elastic keratins of hair have fewer interchain disulfide bridges than the keratins in mammalian fingernails, hooves and claws. Keratins accept a high proportion of the smallest amino acid, Gly, as well as the next smallest, Ala. In the instance of β-sheets, Gly allows sterically-unhindered hydrogen bonding between the amino and carboxyl groups of peptide bonds on adjacent protein chains, facilitating their close alignment and strong binding. Fibrous keratin bondage then twist around each other to course helical filaments.
Elastin, the connective tissue protein, also has a high pct of both glycine and alanine. An insoluble condom-similar protein, elastin confers elasticity on tissues and organs. Elastin is a macromolecular polymer formed from tropoelastin, its soluble precursor. The secondary construction is roughly 30% β-sheets, twenty% α-helices and 50% unordered. The elastic backdrop of natural elastin are attributed to polypentapeptide sequences (Val-Pro-Gly-Val-Gly) in a cross-linked network of randomly coiled chains. H2o is believed to act equally a "plasticizer", assisting elasticity.
Collagen is a major component of the extracellular matrix that supports about tissues and gives cells structure. It has great tensile force, and is the chief component of fascia, cartilage, ligaments, tendons, bone and skin. Collagen contains more than Gly (33%) and proline derivatives (20 to 24%) than practice other proteins, just very trivial Cys. The primary structure of collagen has a frequent repetitive pattern, Gly-Pro-10 (where X is a hydroxyl begetting Pro or Lys). This kind of regular repetition and high glycine content is found in only a few other gristly proteins, such as silk fibroin (75-80% Gly and Ala + x% Ser). Collagen chains are approximately 1000 units long, and assume an extended left-handed helical conformation due to the influence of proline rings. Three such chains are wound near each other with a correct-handed twist forming a rope-like superhelical quaternary structure, stabilized past interchain hydrogen bonding.
Globular proteins are more soluble in aqueous solutions, and are mostly more sensitive to temperature and pH modify than are their fibrous counterparts; furthermore, they practise non accept the loftier glycine content or the repetitious sequences of the fibrous proteins. Globular proteins incorporate a variety of amino acids, many with large side bondage and reactive functional groups. The interactions of these substituents, both polar and nonpolar, often causes the poly peptide to fold into spherical conformations which gives this course its name. In contrast to the structural function played past the gristly proteins, the globular proteins are chemically reactive, serving as enzymes (catalysts), transport agents and regulatory messengers.
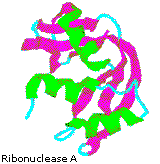 Although globular proteins are generally sensitive to denaturation (structural unfolding), some tin can be remarkably stable. 1 example is the small enzyme ribonuclease A, which serves to digest RNA in our food by cleaving the ribose phosphate bond. Ribonuclease A is remarkably stable. One procedure for purifying it involves handling with a hot sulfuric acid solution, which denatures and partially decomposes most proteins other than ribonuclease A. This stability reflects the fact that this enzyme functions in the inhospitable environment of the digestive tract. Ribonuclease A was the beginning enzyme synthesized past R. Bruce Merrifield, demonstrating that biological molecules are just chemic entities that may be synthetic artificially.
Although globular proteins are generally sensitive to denaturation (structural unfolding), some tin can be remarkably stable. 1 example is the small enzyme ribonuclease A, which serves to digest RNA in our food by cleaving the ribose phosphate bond. Ribonuclease A is remarkably stable. One procedure for purifying it involves handling with a hot sulfuric acid solution, which denatures and partially decomposes most proteins other than ribonuclease A. This stability reflects the fact that this enzyme functions in the inhospitable environment of the digestive tract. Ribonuclease A was the beginning enzyme synthesized past R. Bruce Merrifield, demonstrating that biological molecules are just chemic entities that may be synthetic artificially.
By clicking the cartoon image on the left, an interactive model of ribonuclease A will be displayed.
| Return to Tabular array of Contents |
|---|
This page is the property of William Reusch. Comments, questions and errors should be sent to whreusch@msu.edu.
These pages are provided to the IOCD to assist in capacity building in chemic education. 05/05/2013
![]()
Source: https://www2.chemistry.msu.edu/faculty/reusch/virttxtjml/protein2.htm
Posted by: presleralles1971.blogspot.com


0 Response to "how to draw amino acid sequence"
Post a Comment